Combined percutaneous hepatic perfusion with ipilimumab plus nivolumab in metastatic uveal melanoma (CHOPIN): A single-center, open-label, randomized, phase II study
Ellen Kapiteijn (Leiden, Netherlands), Linde Van den Hoek (Leiden, Netherlands), Frank Speetjens (Leiden, Netherlands), Stéphanie M. Zunder (Leiden, Netherlands), Arian Van Erkel (Leiden, Netherlands), Rutger Van der Meer (Leiden, Netherlands), Carla Van Rijswijk (Leiden, Netherlands), Jacob Lutjeboer (Leiden, Netherlands), Thaïs M. Tong (Dordrecht, Netherlands), Mare Jonker (Leiden, Netherlands), Caroline Roozen (Leiden, Netherlands), Sabine Kropff (Leiden, Netherlands), Els L. Van Persijn van Meerten (Leiden, Netherlands), Christian Martini (Leiden, Netherlands), Remco Zoethout (Leiden, Netherlands), Marianne H. Sitsen (Leiden, Netherlands), Fred Tijl (Leiden, Netherlands), Christian U. Blank (Amsterdam, Netherlands), Mark Burgmans (Leiden, Netherlands)
Disclosures
E. Kapiteijn: Financial Interests, Institutional, Advisory Board: Delcath, Lilly; Financial Interests, Institutional, Invited Speaker: Immunocore; Financial Interests, Institutional, Coordinating PI: BMS, Pierre-Fabre, Delcath, Novartis. L. Van den Hoek: Financial Interests, Institutional, Funding, Part of my PhD is funded by Delcath.: Delcath. M. Burgmans: Non-Financial Interests, Institutional, Product Samples, Delcath Systems offers in-kind contributions (supply of materials free of charge) for the CHOPIN study (PHP +/- immunotherapy): Delcath Systems; Non-Financial Interests, Institutional, Product Samples, BMS offers in-kind contributions (supply of drugs free of charge) for the CHOPIN study (PHP +/- immunotherapy): BMS. All other authors have declared no conflicts of interest.
Background
Metastatic uveal melanoma is a rare cancer with poor prognosis, with a reported median overall survival of around 11 months. Percutaneous hepatic perfusion has shown response rates of 27.3 to 72% in patients with liver-dominant or liver-only metastatic uveal melanoma, but remains ineffective against extrahepatic disease. Immune checkpoint inhibitors ipilimumab and nivolumab demonstrate limited efficacy in uveal melanoma, though retrospective studies suggest improved outcomes when combined with liver-directed therapies.
Methods
In this prospective, investigator-initiated, phase 2 trial, 76 patients with liver-only or liver-dominant metastatic uveal melanoma were randomized 1:1 to receive percutaneous hepatic perfusion alone (perfusion group) or combined with ipilimumab and nivolumab (combination group). The primary endpoint was one-year progression-free survival; secondary endpoints included overall survival, best overall response rate, and safety.
Results
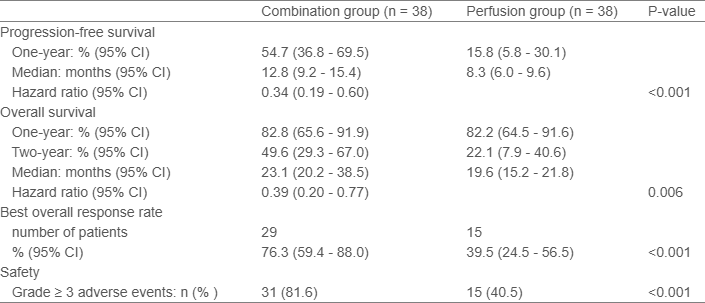
The median follow-up was 24.9 months. The primary endpoint was met, with a one-year progression-free survival of 54.7% (95% CI, 36.8 to 69.5) in the combination group versus 15.8% (95% CI, 5.8 to 30.1) in the perfusion group (P<0.001). The combination also significantly improved overall survival (23.1 vs. 19.6 months, P=0.006), and best overall response rate (76.3% vs. 39.5%, P<0.001). Grade 3 or higher treatment-related adverse events were more frequent in the combination group (81.6% vs. 40.5%, P<0.001), but most were manageable with standard care or self-limiting.
Conclusions
The addition of ipilimumab and nivolumab to percutaneous hepatic perfusion significantly improves progression-free survival, overall survival and best overall response rate, but with manageable toxicity, offering a promising new treatment paradigm for patients with metastatic uveal melanoma.
Clinical trial identification
NCT04283890; EudraCT: 2018-004248-49.
Legal entity responsible for the study
LUMC, Leiden, the Netherlands.
Funding
Delcath, Bristol Myers Squibb.
Disclaimer
© Copyright 2025 European Society for Medical Oncology (ESMO). All rights reserved.
The mention of any company, product, service, or therapy in this collection of materials does not constitute an endorsement of any kind by ESMO. It is the responsibility of the treating physician or other health care provider, relying on independent experience and knowledge of the patient, to determine drug dosages and the best treatment for the patient. Viewers are advised to check the appropriate medical literature and the product information currently provided by the manufacturer of each drug to be administered to verify, among other matters, the dosage, method, and duration of administration, or contraindications. Viewers are also encouraged to contact the manufacturer with questions about the features or limitations of any products. ESMO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of the material contained in this publication or to any errors or omissions.